Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Ring Models of Atoms and Molecules with Two Opposite Spins of Proton and Neutron Rings
*Corresponding author:Pavel Ošmera, Mechanical Department, Brno University of Technology, retired university professor, Czech Republic.
Received:April 04, 2023;; Published:April 21, 2023
DOI: 10.34297/AJBSR.2023.18.002497
Abstract
Using the ring model of atoms and molecules, it was revealed that there must be two types (type A and type B) of atoms that differ in the direction of rotation of individual rings (for type B, all rings rotate opposite to the directions of rotation for type A). The rotations of protons and neutrons in atoms are given by a system that resembles a “cogwheel” in gear box.
Keywords: Ring model, Direction of Rotation of proton, Neutron and Electron, Directions of magnetic moments, Photocatalytic cleaner with TiO2
Introduction
The solution to this problem was found in the design of the TiO2 model, which has a total of 22 protons, 22 electrons and 26 neutrons in titanium atom (Ti). Each oxygen atom has 8 protons, 8 electrons and 8 neutrons. The TiO2 model therefore consists of 118 rings (protons, neutrons and electrons). Two types of electrons are distinguished in the model (valence electrons are colored light blue and inner electrons are colored dark blue). The direction of rotation of the proton determines the direction of the magnetic moment that exits the atom. For an external view of the proton, it has (for clockwise rotation of the proton) the proton has a magnetic moment that exits the proton (yellow arrow). For counter clockwise rotation of the proton) the proton has a magnetic moment that enters the proton (green arrow). The article uses the knowledge described in [1-8].
Model of the A-Type and B-Type Oxygen Atom
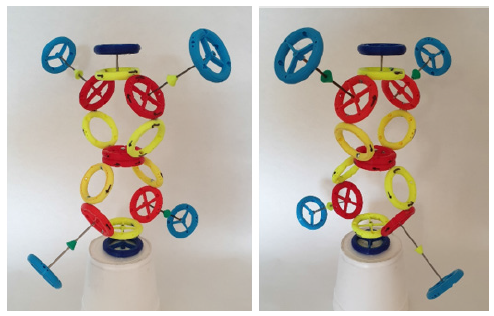
In (Figure 1) is a topological model of oxygen of type A. The topological model follows the mutual connections of the rings in the atom and molecule, while not observing the correct dimensions and distances of the individual rings. For example: at the drawn size of the proton, the electron would be far away on the model (it would not fit in the A4 format). On the contrary, with the correct size of the distance between the electron and the proton, the proton would be a dot in the picture (the nucleus of the atom would also be a dot) (Figure 1,2).
Oxygen Molecule O2
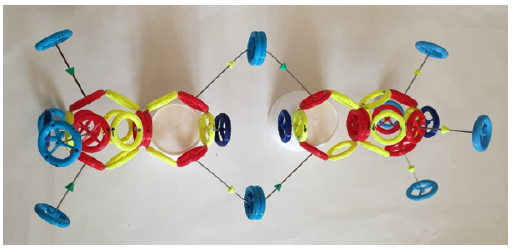
In the case of an oxygen molecule, oxygen atoms of type A and type B are joined. The magnetic moments in the binding of two protons must follow the same direction. Only the magnetic moment with the yellow arrow can be combined with the magnetic moment with the green arrow. The hydrogen molecule is in (Figure 3).
Name of Electrons Inside Atom of Oxygen
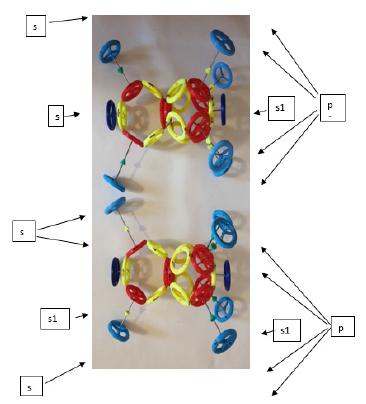
(Figure 4).
Type A and Type B 3 of Carbon Atoms
(Figure 5) shows both versions of the carbon atom (type A and type B). Since the carbon atom is centripetal, we also get type B from type A by rotating the type A model by 180 degrees.
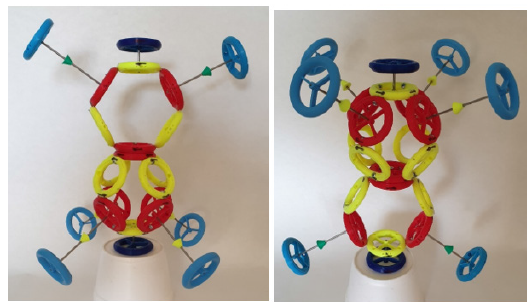
Figure 1:Topological model of an A-type oxygen atom (red rings are protons, yellow are neutrons, blue are electrons, yellow and green arrows are directions of magnetic moments).
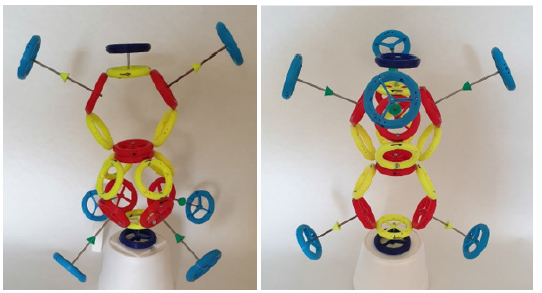
Figure 2:Topological model of the B-type oxygen atom (red rings are protons, yellow are neutrons, blue are electrons, yellow and green arrows are directions of magnetic moments).
Carbon Dioxide CO2 Molecule
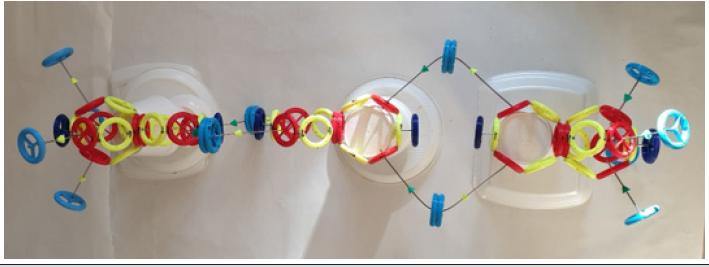
(Figure 6) shows a model of the carbon dioxide molecule, which consists of a carbon atom and two oxygen atoms of both types. Carbon dioxide has both types of oxygen atom in the molecule. Two oxygen atoms of the same type cannot form a carbon dioxide molecule. Molecule CO2 has linear structure.
Titanium Dioxide TiO2 Molecule
(Figure 7) shows a model of titanium atom and the titanium dioxide molecule, which consists of a titanium atom and two oxygen atoms of both types. Titanium dioxide has both types of oxygen atom in the molecule. Two oxygen atoms of the same type cannot form a carbon dioxide molecule. TiO2 molecules have linear structure as CO2 molecule. TiO2 molecules are used in photocatalytic cleaners. The surface with TiO2 is activated using UV light (band A – 365 nm) emitted by UV LEDs.
Photocatalytic Cleaner with Titanium Dioxide
In (Figure 8) is a look inside the photocatalytic air cleaner 2HELICO, manufactured by NCT s.r.o. (New Czech Tech. Ltd.). Using the dog cleaner (purifier) breaks down complex molecules (viruses, bacteria, fungi, cat and dog odors, cigarette smoke) into harmless substructures.
Conclusion
The aim of the article was to show the necessity that there must exist for each atom both types (type A and type B) in which all the rings rotate in the opposite direction. Therefore, protons have magnetic moments with opposite directions, which enables them to combine into molecules. Only protons that have opposite directions of magnetic moments can be connected (only connecting the direction with the green arrow to the direction with the yellow arrow). We can call this direction: spin in and spin out. Knowledge of structure rules of the atomic nucleus and the properties of vortex electromagnetic field allow us to create relatively precisely the structures of individual atoms and molecules. Properties of atoms are largely described by the structure of their electron shells. However, the standard model of atoms does not allow define this structure exactly. New theory VFRT (Vortex-Fractal-Ring-Theory) can solve this lack. Theory VFRT uses fractal ring structure of the electron, the proton and the neutron, and can describe the inner structure of atomic nuclei. Fractal descriptions of Nature are very promising. The atomic nucleus can be built from ring protons and neutrons. This new theory assumes that the arrangement of electron shells arises from the structure of the atomic nucleus. Electrons are not in orbit around the atomic nucleus, but each electron levitates with the corresponding proton of the nucleus. The levitation bond between the electron and the proton is formed by an electromagnetic vortex structure. Theory VFRT expands understanding of nature through a new perspective on the evolution of lifeless nature using a vortex, fractal ring substructures with self-organization, from quarks, electrons, protons and neutrons, atoms, molecules, to the structure of complex organic compounds.
Acknowledgement
None.
Conflict of Interest
None.
References
- Pauling L (1988) General Chemistry, Dover publication, Inc, New York.
- Ramsden EN (2000) A-Level Chemistry, Nelson Thornes Ltd, fourth edition.
- Ošmera P (2012) Fractal dimension of electron. published in the Proceedings of MENDEL p186-191.
- Ošmera P (2015) Ring structures of atoms and molecules. San Diego, California, USA.
- Ošmera P (2017) RING STRUCTURES OF MATTER. published in the Proceedings of NANOCON2017, Brno, Czech Republic.
- Ošmera P, Werner P (2015) Ring structure of atoms and molecules. Proceedings of SPIE Vol.95701C-1, San Diego.
- Werner P (2018) Fundamentals of modeling the ring structure of elementary particles of matter. Institute of Theoretical and Experimental Electrical Engineering, Brno, ISBN 978-80-214-5620-4.
- More about VFRT theory can be found on http://www.pavelosmera.cz.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.